
Keynote 057 plus#
The sBLA is based on data from the KEYNOTE-859 trial, in which KEYTRUDA plus chemotherapy demonstrated a statistically significant improvement in overall survival (OS) versus chemotherapy alone, regardless of PD-L1 expression, in patients who were human epidermal growth factor receptor 2 (HER2) negative.
Keynote 057 license#
Food and Drug Administration (FDA) has accepted for review a new supplemental Biologics License Application (sBLA) seeking approval for KEYTRUDA, Merck’s anti-PD-1 therapy, in combination with fluoropyrimidine- and platinum-containing chemotherapy, for the first-line treatment of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma.
RAHWAY, N.J., April 13, 2023-( BUSINESS WIRE)-Merck (NYSE: MRK), known as MSD outside of the United States and Canada, today announced the U.S. In a press release, Merck stated that its application will be reviewed by the FDA’s Oncologic Drugs Advisory Committee on December 17, 2019, and that it anticipates a Prescription Drug User Fee Act, or target action date, in January 2020.Acceptance based on results from the Phase 3 KEYNOTE-859 trial, which showed significant overall survival benefit in these patients with HER2-negative disease, regardless of PD-L1 expression Keytruda was previously approved as a single agent or in combination with other agents for several other types of cancer, including melanoma, Merkel-cell carcinoma, lung cancer, hepatocellular carcinoma, head and neck cancer, Hodgkin lymphoma, primary mediastinal large B-cell lymphoma, urothelial carcinoma, gastric cancer, esophageal cancer, cervical cancer, and renal-cell carcinoma. Patients with high-grade non–muscle-invasive bladder cancer are at high risk for developing muscle-invasive and metastatic disease. Approximately 75% of these patients are diagnosed with non–muscle-invasive bladder cancer, which means that the cancer cells have not grown into the main muscle layer of the bladder. It is estimated that >80,000 new cases of bladder cancer will be diagnosed in 2019 in the United States. Patients received 200 mg of pembrolizumab every 3 weeks for up to 24 months or until unacceptable toxicity, progressive disease, or detection of persistent or recurrent high-risk non–muscle-invasive bladder cancer. Major efficacy outcome measures were complete response and duration of response.
Keynote 057 trial#
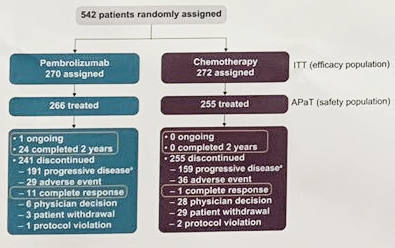
The filing was based on data from the multicenter, open-label, single-arm, phase 2 KEYNOTE-057 clinical trial of 102 patients (median age, 73 years) with Bacillus Calmette-Guerin–unresponsive, high-risk, non–muscle-invasive bladder cancer with carcinoma in situ with or without papillary tumors who were ineligible for or had decided not to undergo cystectomy. If approved, the drug would be indicated as monotherapy to treat patients with Bacillus Calmette-Guerin–unresponsive, high-risk, non–muscle-invasive bladder cancer who are ineligible for or have decided not to undergo cystectomy. On December 2, 2019, Merck announced that the FDA has granted priority review for its supplemental biologics license application for the anti–PD-1 agent pembrolizumab (Keytruda).


 0 kommentar(er)
0 kommentar(er)
